Case Report
Patient’s
Identity
|
Name
|
Mr. A
|
|
Age
|
38 years
|
|
Gender
|
Male
|
Autoanamnesis
(5 August 2015)
Chief Complain
Since 2 days before admitted to Arifin Achmad’s
Hospital, Patient complain weaked and numbed on his two legs after fell from
roof
Present illness history
§ 2
days before admitted to Arifin Achmad Hospital, patient fell from roof with
height approximately ± 5 m. Patient fell from the roof when repaired the tile
with supine position which the back first get the trauma and lifted the head
upward. After that, patient complain weaked (can’t moved and lifted his leg to
stand up) and numbed on his two legs from waist to toe. Unconscious (-),
Headache (-), nausea (-), gag (-). Patient immediately refered to Pematang
Rebah Hospital and got a medical management. 30 minutes later, weaked and
numbed only patients felt on his two legs from knee to toe until now.
§ At
Pematang Rebah Hospital, patient still complained weaked and numbed on his two
legs from knee to toe. Patient also complained pain if his leg was held and
complained no sensation to urination and defecate. Patient also can’t urination
dan defecate. The first time he can
urinate after 22 hours from that accident but still can’t defecate. Patient then
referred to Arifin Achmad Hospital.
Past Illness history
§ History
of brain trauma (-)
§ History
numb of his leg and arm (-)
§ History
of last fever (-)
§ History
of stroke (-)
§ Diabetes
Mellitus (-)
§ Hypertensi(-)
Family Illness history
(-)
RESUME ANAMNESIS
Mr.A,
38 years old admitted to hospital at 4 August 2015 referred from pematang rebah
hospital with weaked and numbed on his two legs after fell from
roof since 2 days. Patient fell from the roof when reparied the tile with
supine position which the back first get the trauma with the head upward.
Unconscious (-), Headache (-), nausea (-), gag (-). History last fever(-).
Patient also complained pain in his leg if held and no sensation to urination
and defecate. Patient also can’t urination dan defecate.
III. Physical Examination
A.
Generalized
Condition
Blood Presure : 130/90
mmHg
Heart Rate : 68
bpm
Respiratory : Respiratory
rate : 21 x/mnt Type : abdominotorakal
Temperature : 36,9°C
Weight : 60 Height : 162 IMT : 22,8 (Normal)
B.
Neurological
status
Consciousness : Composmentis GCS : 15 (E4
V5 M6)
Noble Function : Normal
Neck Rigidity : Negatif
Cranial
Nerve : Normal
Motoric : Paraparese with
flaccid type
Sensory
: Hipestesia dermatom L4-S3 in right and left leg
with
Parestesia
Position,
Two point discrimination (Normal)
Stereognosis,
Graphestesia (Normal)
Coordination : Normal
Otonom : Abnormality of defecate and urination
Reflex
Fisiologis : Areflex of achilles
reflex and patella reflex
Patologic : No patologic reflex
V. WORKING DIAGNOSIS :
CLINIC DIAGNOSIS : Spinal Shock
-
Paraparese with flaccid type
-
Hipestesi dermatom L4-S3 in right and left leg with
parestesia
-
Abnormality of defecate and urination
-
Areflex of Patella reflex and achilless
reflex
TOPIC DIAGNOSIS :
L2 medulla spinalis
anterior cord
ETIOLOGIC DIAGNOSIS : Trauma Vetebrae
DIFFERENTIAL DIAGNOSIS:
Radicular injury
SUGGESTION EXAMINATION :
1.
Blood
Routine
2.
Blood
Chemistry
3.
Electrolit
4.
X-Ray
Rontgen LumboSakral AP-Lateral
5. MRI Thoraco Lumbal Vertebrae
MANAGEMENT :
§ IVFD
RL20 dpm
§ Inj.
Ketorolac 3 x 30 mg
§ Inj Ranitidin
2 x 50 mg
§ Inj
Methylprednisolone 4 x 125 mg
§ Vit B Complex 1 x 1
tab (p.o)
§ Immobilisation
LABORATORIUM FINDING : Normal
AP/L LumboSakral X- Ray
Compression
Fracture Vetebrae L1
Osteofit Vetebrae
L3-
MRI THORACO LUMBAL
Brusting fracture with bone edema corpus vetebrae L1
Kontusio Conus Medullaris
Spondylo-lisis Th12-L1
Final diagnose : Mielopaty lumbal ec Spinal Cord Injury
Discussion
Spinal
Cord Injury
11.Anatomy of spine, spinal cord,
demartomes and miotomes
a.
Spine
The spine (vertebral
column) bears the weight of the head, neck, trunk and upper extremities. Its
flexibility is greatest in the cervical region, intermediate in the lumbar
region, and lowest in the thoracic region. Its uppermost vertebrae (atlas and
axis) articulate with the head, and its lower most portion, the sacrum (which
consists of 5 vertebrae fused together), articulates with the pelvis. There are
7 cervical, 12 thoracic, and 5 lumbar vertebrae, making a
total of 24 above the sacrum. Below the
sacrum, the coccyx is composed of 3 to 6 coccygeal vertebrae.1
b.
Spinal
cord
Like the brain, the spinal cord is intimately enveloped by the piamater,
which contains numerous nerves and blood vessels; the piamater merges with the
endoneurium of the spinal nerve rootlets and also continues below the spinal
cord as the filum terminale internum. The weblike spinal arachnoid
membrane contains only a few capillaries and no nerves. The denticulate
ligament runs between the piamater and the duramater and anchors the spinal
cord to the duramater. The spinal duramater originates at the edge of
the foramen magnum and descends from it to form a tubular covering around the
spinal cord. Its lumen ends at the S1–S2 level, where it continues as the filum
terminale externum, which attaches to the sacrum, thus anchoring the dura mater
inferiorly. The duramater forms sleeves around the anterior and posterior
spinal nerve roots which continue distally, together with the arachnoid
membrane, to form the epineurium and perineurium of the spinal nerves. The root
filaments (rootlets) that come together to form the ventral and dorsal
spinal nerve roots are arranged in longitudinal rows on the lateral surface of
the spinal cord on both sides. The ventral root carries onlymotor
fibers, while the dorsal root carries only sensory fibers. There are, therefore, just as many pairs of nerves in each of
these regions as there are vertebrae (12 thoracic, 5 lumbar, 5 sacral and 1
coxcigeal).1,2
c.
Dermatomes
and miotomes
A dermatome is defined as the cutaneous area whose sensory
innervation is derived from a single spinal nerve (i.e., dorsal root). The
division of the skin into dermatomes reflects thesegmental organization of the
spinal cord and its associated nerves.1
|
Picture 1 Relation of Spinal nerve roots to
vetebraes
|
Picture 2 Dermatomes and myotomes of Spinal
roots
12.
Epidemiology
Acute spinal cord
injury (SCI), whether traumatic or nontraumatic in etiology, has a tremendous
cost not only for patients and families, but also for society as a whole. The
incidence of SCI in the United States is estimated to be 30–40 cases per 1
million inhabitants. However, an exact incidence is difficult to ascertain
because SCI is not reportable and there have not been large prospective and
comprehensive studies done since the 1970s. There are 8000–10,000 new cases of
acute SCI a year. It does appear, however, that despite the currently available
acute and emergency care as well as the preventive measures the incidence of
SCI and disorders and the resulting disabilities have remained stable.
Approximately 183,000–230,000 people are living today with SCI. The average age
of acute SCI is 31.8 yr and 80% of those affected are male.4
23.
Etiology
a.
Traumatic
spinal cord injury
The most common causes
of traumatic SCI include motor vehicle accident (MVA), motorcycle accidents
(MCA), and falls. Other causes are work-related injuries, recreation, and
sports activities such as diving injuries, and penetrating injuries secondary
to knife or gunshot wounds. The most common location of SCI is the cervical
spine (C5–C6 level) followed by the thoracolumbar junction, thoracic, and
lumbar spine. Lap belt injuries are associated with thoracolumbar flexion
distraction injury. Multiple level injuries occur in as high as 20% of the
cases.4,5
b.
Nontraumatic
spinal cord injury
Nontraumatic
etiologies leading to an acute SCI include various bacterial, viral, fungal, or
parasitic infections; tumors or other compressive lesions of the spinal cord;
vascular events such as infarction; demyelinating lesions in spinal multiple
sclerosis; toxins; autoimmune disorders; or nutritional deficiencies such as
pernicious anemia (Table 1).4,5
Table 1. Etiologies Nontraumatic
spinal cord injury4
|
Acute
|
Subacute–chronic
|
Chronic
|
|
Acute transverse myelitis
Spinal epidural abscess
Spinal epidural hematoma
Spinal cord infarction
Functional
|
Spinal cord tumor
Radiation myelopathy
Vascular malformation (AVM)
Infectious
Nutritional: vitamin B12 deficiency
|
Syringomyelia
Multiple sclerosis
Lumbar canal stenosis
Cervical spondylosis
Amyotrophic lateral Sclerosis
|
34.
Mechanisms of injury
It is
generally accepted that acute SCI is a two step process that involves primary
and secondary mechanisms. The primary mechanism results from the initial
mechanical injury due to local deformation and energy transformation, whereas
the secondary mechanisms encompass a cascade of biochemical and cellular
processes that initiated by the primary process and cause ongoing cellular
damage and death.6,7
Primary SCI is most commonly a
combination of the initial impact as well as subsequent persisting compression.
The most frequent primary mechanism of SCI is impact of bone and ligament
against the spinal cord from high translational forces, such as that generates
by flexion, extension, axial rotation, or vetebral compression. The spinal cord
may consequently be compressed, stretched, or crushed by fracture or
dislocations, burst fractures of the vertebral body, or acutely ruptured
intervertebral discs.
A useful classification of spinal
injuries is one that divides them into fracture-dislocations, pure
fractures, and pure dislocations. The relative frequency of these types is
about 3:1:1. All three types of spinal injury previously mentioned are produced
by a similar mechanism, usually a vertical compression of the spinal column to
which anteroflexion is added; or, the mechanism may be one of vertical
compression and retroflexion (commonly referred to as hyperextension). The most
important variables in the mechanics of vertebral injury are the structure of
the bones at the level of the injury and the intensity, direction, and point of
impact of the force.7
An understanding of the mechanism of injury and the
radiographic findings can provide insight into the biomechanical stability of
the vetebral column after SCI. For example, In the case of severe forward flexion
injury, the head is bent sharply forward when the force is applied. The
adjacent cervical vertebrae are forced together at the level of maximum stress.
The anteroinferior edge of the upper vertebral body is driven into the one
below, sometimes splitting it in two. The posterior part of the fractured body
is displaced backward and compresses the cord. Concomitantly, there is tearing
of the interspinous and posterior longitudinal ligaments. Less severe degrees
of anteroflexion injury produce only dislocation. Vulnerability to the effects
of anteroflexion (and to some extent to retroflexion injuries) is increased by
the presence of cervical spondylosis or ankylosing spondylitis or by a
congenital stenosis of the spinal canal.6,7
In hyperextension injuries, the mechanism is
one of vertical compression with the head in an extended position. Stress is
mainly on the posterior elements (the laminae and pedicles) of the midcervical
vertebrae (C4 to C6), which may be fractured unilaterally or bilaterally—and on
the anterior ligaments. This dual disruption in the spinal architecture allows
for displacement of one vertebral body on the adjacent one. The dislocation
results in the cord being caught between the laminae of the lower vertebra and
the body of the one above.6,7
Secondary mechanisms that result from biochemical
cascades that occur after the initial event are a source of ongoing SCI and
neurologic deterioration. These cause damage to neural tissue onn the cellular
level and include the pathologic effects of microvaskular changes, excitatory
amino acids, cell membrane destabilization, free radicals, inflammatory
mediators, and neroglia apoptosis.6
Table 2. Spinal Fractures1
|
Fracture/Dislocation
|
Pathogenesis
|
Stability1
|
|
Cervical spine
|
||
|
Atlantoaxial dislocation
Jefferson’s fracture2
Dens fracture
Bilateral axis arch fracture4
Dislocation fracture of C3–7
Lateral compression fracture
|
Dislocation between C1 and C2
Axial trauma
Hyperflexion
Hyperflexion and distraction
Hyperflexion
Flexion and axial compression
|
Unstable
Unstable
Unstable3
Unstable
Unstable
Stable
|
|
Thoracic spine, lumbar spine
|
||
|
Compression fracture
Burst fracture
Dislocation fracture
|
Fall
(back, buttocks, extended legs),direct trauma. These fractures may be pathological
(osteoporosis, myeloma,
metastasis)
|
Stable
Stable
Unstable
|
1 At the time of injury. 2 Fracture of the ring of C1
due to compression between the occiput and C2. 3 May be
overlooked if the dens is not
displaced; sometimes stable. 4 Hangman’s fracture.
45.
Clinical
effects of spinal cord injury
When the spinal cord is suddenly and virtually or
completely severed, three disorders of function are at once evident: (1) all
voluntary movement in parts of the body below the lesion is immediately and
permanently lost; (2) all sensation from the lower (aboral) parts is abolished;
and (3) reflex functions in all segments of the isolated spinal cord are
suspended. It is of variable duration (1 to 6 weeks as a rule but sometimes far
longer) and is so dramatic that Riddoch used it as a basis for dividing the clinical
effects of spinal cord transection into two stages, that of spinal shock and
areflexia followed by the stage of heightened reflex activity.
a.
Stage of Spinal Shock
or Areflexia
The loss of motor function at the time
of injury—tetraplegia with lesions of the fourth to fifth cervical segments or
above, paraplegia with lesions of the thoracic cord—is accompanied by immediate
atonic paralysis of bladder and bowel, gastric atony, loss of sensation below a
level corresponding to the spinal cord lesion, muscular flaccidity, and almost
complete suppression of all spinal segmental reflex activity below the lesion.
As a result of their sudden separation from higher levels, the neural elements
below the lesion fail to perform their normal function. Also impaired in the
segments below the lesion is the control of autonomic function. Vasomotor tone,
sweating, and piloerection in the lower parts of the body are temporarily
abolished. Systemic hypotension may be severe and contribute to the spinal cord
damage.7
The sphincters of the bladder and the
rectum remain contracted to some degree due to the loss of inhibitory influence
of higher central nervous system (CNS) centers, but the detrusor of the bladder
and smooth muscle of the rectum are atonic. Urine accumulates until the
intravesicular pressure is sufficient to overcome the sphincters; then driblets
escape (overflow incontinence). There is also passive distention of the bowel,
retention of feces, and absence of peristalsis (paralytic ileus). Genital
reflexes (penile erection, bulbocavernosus reflex, contraction of dartos
muscle) are abolished or profoundly depressed.7
b. Stage of
Heightened Reflex Activity
This is the more
familiar neurologic state that emerges within several weeks or months after
spinal injury. Usually, after a few weeks, the reflex responses to stimulation,
which are initially minimal and unsustained, become stronger and more easily
elicitable and as time passes come to include additional and more proximal
muscles.7
Various combinations of residual deficits (of lower
and upper motor neurons and sensory neurons) are to be expected. Some of the
resulting clinical pictures are complete or incomplete voluntary motor
paralysis; a flaccid atrophic paralysis of upper limb muscles (if appropriate
segments of gray matter are destroyed) with spastic weakness of the legs; a
partial or rarely a complete Brown-Se´quard syndrome; and each of these occurs
with variable sensory impairment in the legs and arms. High cervical lesions
may result in extreme and prolonged tonic spasms of the legs due to release of
tonic myotatic reflexes. Under these circumstances, attempted voluntary
movement may excite intense contraction of all flexor and extensor muscles
lasting for several minutes. Segmental damage in the low cervical or lumbar
gray matter, destroying inhibitory Renshaw neurons, may release activity of
remaining anterior horn cells, leading to spinal segmental spasticity. Any
residual symptoms persisting after 6 months are likely to be permanent,
although in a small proportion of patients some return of function
(particularly sensation) is possible after this time. Loss of motor and sensory
function above the lesion, coming on years after the trauma, occurs
occasionally and is due to an enlarging cavity in the proximal segment of the
cord.7
Table 3. The
neurological deficits depend on the level of the lesion1
|
Level
|
Motor Deficit
|
Sensory Deficit
|
Autonomic Deficit
|
|
C1–C3
|
Quadriplegia,
neck muscle paresis, spasticity, respiratory paralysis
|
Sensory
level at back of head/edge of lower jaw; pain in back of head, neck, and
shoulders
|
Voluntary
control of bladder, bowel, and sexual function replaced by reflex control;
Horner syndrome
|
|
C4–C5
|
Quadriplegia,
diaphragmatic breathing
|
Sensory
level at clavicle/shoulder
|
Same as above
|
|
C6–C8
|
Quadriplegia,
spasticity, flaccid arm paresis, diaphragmatic breathing
|
Sensory
level at upper chest wall/back; arms involved, shoulders spared
|
Same as above
|
|
T1–T5
|
Paraplegia,
diminished respiratory volume
|
Sensory
loss from inner surface of lower arm, upper chest wall, back region downward
|
Voluntary
control of bladder, bowel, and sexual function replaced by reflex control
|
|
T5–T10
|
Paraplegia, spasticity
|
Sensory
level on chest wall and back corresponding to level of spinal cord injury
|
Same as above
|
|
T11–L3
|
Flaccid paraplegia
|
Sensory
loss from groin/ventral thigh downward, depending on level of injury
|
Same as above
|
|
L4–S2
|
Distal flaccid paraplegia
|
Sensory
loss at shin/dorsum of foot/posterior thigh downward, depending on level of
injury
|
Flaccid
paralysis of bladder and bowel, loss of erectile function
|
|
S3–S5
|
Distal flaccid paraplegia
|
Sensory
loss in perianal region and inner thigh
|
Flaccid
paralysis of bladder and bowel, loss of erectile function
|
56.
Neurologic
Assestment and classification
The
level of the spinal cord and vertebral lesions can be determined from the
clinical findings. Diaphragmatic paralysis occurs with lesions of the upper
three cervical segments (an unrelated transient arrest of breathing is common
in severe head injury). Complete paralysis of the arms and legs usually
indicates a fracture or dislocation at the fourth to fifth cervical vertebrae.
If the legs are paralyzed and the arms can still be abducted and flexed, the
lesion is likely to be at the fifth to sixth cervical vertebrae. Paralysis of
the legs and only the hands indicates a lesion at the sixth to seventh cervical
level. The spinal cord ends at the first lumbar vertebra, usually at its
rostral border. Vertebral lesions below this point give rise predominantly to cauda
equina syndromes; these carry a better prognosis than injuries to the lower
thoracic vertebrae, which involve both cord and multiple roots
The level of sensory loss on the trunk, determined
by perception of pinprick, is an accurate guide to the level of the lesion,
with a few qualifications. Lesions of the lower cervical cord, even if
complete, may show a sparing of sensation down to the nipple line because of
the contribution of the C3 and C4 cutaneous branches of the cervical plexus,
which variably innervate skin below the clavicle. Or, a lesion that involves
only the outermost fibers of the spinothalamic pathways, sparing the innermost
ones, results in a sensory level (to pain and temperature) well below the level
of the lesion. In all cases of spinal cord and cauda equina injury, the
prognosis for recovery is more favorable if any movement or sensation is
elicitable during the first 48 to 72 h.7
A neurologic examination with detailed recording of
motor, sensory, and sphincter function is necessary to follow the clinical
progress of spinal cord injury. Common practice is to define the injury
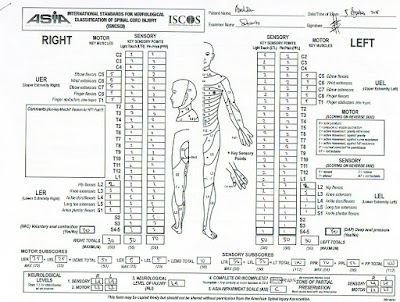
according to the standards of the American Spinal Injury Association (ASIA).4-8
Table 4. ASIA Impairment Scale (AIS) (modified from
Frankel)
|
A = Complete
|
No sensory
or motor function is preserved in the sacral segments S4-S5
|
|
B = Sensory
incomplete
|
Sensory but
not motor function is preserved below the neurological level and includes the
sacral segments S4-S5, AND no motor function is preserved more than three
levels below the motor level on either side of the body.
|
|
C = Motor
incomplete
|
Motor
function is preserved below the neurological level**, and more than half of
key muscle functions below the single neurological level of injury have a
muscle grade less than 3 (Grades 0–2).
|
|
D = Motor
incomplete
|
Motor function is preserved below the neurological level**,
and at least half (half or more) of key muscle functions below the NLI have a
muscle grade >3.
|
|
E = Normal
|
If sensation and motor function as tested with the
ISNCSCI are graded as normal in all segments, and the patient had prior
deficits, then the AIS grade is E. Someone without a SCI does not receive an
AIS grade.
|
**For an
individual to receive a grade of C or D, i.e. motor incomplete status, they
must have either (1) voluntary anal
sphincter contraction or (2) sacral sensory
sparing (at S4/5 or DAP) with sparing of motor function more than three levels
below the motor level for that side of the body. The Standards at this time
allows even non-key muscle function more than 3 levels below the motor level to
be used in determining motor incomplete status (AIS B versus C).
11. Determine
sensory levels for right and left sides.
22. Determine
motor levels for right and left sides.
Note: in
regions where there is no myotome to test, the motor level is presumed to be
the same as the sensory level, if testable motor function above that level is
also normal.
33.
Determine the single neurological level. this is the
lowest segment where motor and sensory function is normal on both sides, and is
the most cephalad of the sensory and motor levels determined in steps 1 and 2.
44.
Determine whether the injury is Complete or Incomplete
(i.e. absence or presence of sacral sparing)
If voluntary
anal contraction = No AND all S4-5 sensory
scores = 0 AND deep anal pressure = No,
then injury is COMPLETE. Otherwise, injury is Incomplete.
55.
Determine ASIA Impairment Scale (AIS) Grade:
While
not a part of the International Standards examination or AIS classification, these incomplete
syndromes have previously been described in this booklet, and as such have been
maintained.8
a. Central cord syndrome
Central cord
syndrome is the most common of the clinical syndromes, often seen in
individuals with underlying cervical spondylosis who sustain a hyperextension
injury (most commonly from a fall); and may occur with or without fracture and
dislocations. This clinically will present as an incomplete injury with greater
weakness in the upper limbs than in the lower limbs.8
Picture
4 Central cord syndrome5
b. Brown-Sequard syndrome
Brown-Sequard syndrome (historically related to a knife wound) represents a spinal cord hemisection in its pure form, which results in ipsilateral loss of propioception and vibration and motor control at and below the level of lesion, sensory loss of all modalities at the level of the lesion, and contralateral loss of pain and temperature sensation. This specific syndrome in its pure form is rare, more often resulting in a clinical examination with some features of the Brown-Sequard and central cord syndrome. Some refer to this variation as Brown-Sequard-Plus Syndrome.8
Picture
5 Brown-Sequard Syndrome5
c. Anterior cord syndrome
The anterior
cord syndrome is a relatively rare syndrome that historically has been related
to a decreased or absent blood supply to the anterior two-thirds of the spinal
cord. The dorsal columns are spared, but the corticospinal and
spinothalamic tracts are compromised. The clinical symptoms include a loss of
motor function, pain sensation and temperature sensation at and below the
injury level with preservation of light touch and joint position sense.8
Picture
6 Anterior Cord Syndrome5
d. Cauda equina syndrome
Cauda Equina syndrome involves the lumbosacral nerve roots of the cauda equina and may spare the spinal cord itself. Injury to the nerve roots, which are, by definition, lower motor neurons, will classically produce a flaccid paralysis of the muscles of the lower limbs (muscles affected depend upon the level of the injury), and areflexic bowel and bladder. All sensory modalities are similarly impaired, and there may be partial or complete loss of sensation. Sacral reflexes i.e. bulbocavernosus and anal wink will be absent.8
Picture 7 Cauda Equina Syndrome5
e. Conus medullaris syndrome
Conus
Medullaris Syndrome may clinically be similar to the Cauda Equina Syndrome, but
the injury is more rostral in the cord (L1 and L2 area), relating to most
commonly a thoraco-lumbar bony injury. Depending on the level of the lesion, this
type of injury may manifest itself with a mixed picture of upper motor neuron
(due to conus injury) and lower motor neuron symptoms (due to nerve root
injury). In some cases, this may be very difficult to clinically distinguish
from a cauda equina injury. Sacral segments may occasionally show preserved
reflexes (i.e. bulbocavernosus and anal wink) with higher lesions of the conus
medullaris.8
Picture
8 Conus Medullaris Syndrome5
17.
Imaging Studies
Radiologic examinations are undertaken to determine
the alignment of vertebrae and pedicles, fracture of the pedicle or vertebral
body, compression of the spinal cord or cauda equina due to malalignment, or
bone debris in the spinal canal, and the presence of tissue damage within the
cord. The MRI is ideally suited to display these processes but if it is not available
myelography with CT scanning is an alternative. Instability of the spinal
elements can often be inferred from dislocations or from certain fractures of
thepedicles, pars articularis, or transverse processes, but gentle flexion and
extension of the injured areas must sometimes be undertaken and plain films
obtained in each position.7
28.
Management of
spinal cord injury
a.
Acute
medical management
Initial management of SCI patients consists of
establishing airway, breathing, and circulation (ABCs). If endotracheal
intubation is required, the nasotracheal approach with a fiberoptic
bronchoscope is preferred to minimize damage with hyperextension of the neck.
After trauma, any region of the spine suspicious for injury must be immobilized
at the scene. A combination of rigid cervical collar and supportive blocks with
backboard with straps is sufficient. Sandbags and tapes are not recommended.
The immediate aim of treatment is to prevent the secondary damage to the spinal
cord and nerve tissue. Anything that can hinder circulation or oxygenation to
the cord can lead to further damage such as hypotension, hypoxia, hypoxia,
hyperpyrexia, or compression from bony fragments. The possibility of
hemodynamic instability, secondary to ongoing bleeding not identified before
mobilization, or autonomic dysfunction should be noted. Intravenous fluids and
pressors should be immediately available.4,5,6,7
Once the patient enters the hospital, it
is critical that an initial neurological assessment be made. The exam should be
comprehensive enough to allow for proper identification of patients according
to the American Spinal Injury Association (ASIA) standard classification of
SCI and obtain the initial functional
independence measure score (FIM score) (usually performed after the emergent evaluation
and treatment). The former includes sensory examination with light touch and
pin prick of 28 areas as well as strength testing of 10 upper and lower
extremity muscles using a 0–5 score for complete loss to normal strength. After
completing the examination using the ASIA criteria, the injury is determined to
be either complete or incomplete. SCI is deemed complete when there is no motor
or sensory function preserved below the level of injury or in the sacral
segment (S4–5). The level of injury is the most caudal level intact on
examination. The most important medical intervention that can be performed on
the patient with an SCI is the prevention of further injury. This can be
accomplished with vigilance and adherence to spinal protocols.4,5,6
High dose methylprednisolone is considered a
neuroprotective agent in SCI and has been the standard of care for the past 15
years. Methylprednisolone is thought to improve spinal cord function by
inhibiting lipid peroxidase and free radical production. Methylprednisolone was
demonstrated to be an effective therapy for acute SCI in the National Acute
Spinal Cord Injury Study (NASCIS) 2. The NASCIS 1 failed to demonstrate
improvement after a single dose of methylprednisolone, but this dose was much
lower. Patients in NASCIS 2 were treated with a bolus of 30 mg/kg body weight
over 15 min and followed by infusion of 5.4 mg/ kg of body weight for 23 h.
Only the patients started on treatment within 8 h of injury had improvement in
both motor and sensory functioning. If therapy was begun more than 8 h after
SCI, then therenwas no statistically significant improvement. The NASCIS 3
demonstrated improvement if the duration of methylprednisolone therapy was
increased to 48 h for those patients started between 3 and 8 h after injury.
The prior treatment regimen of 24 h should be continued in those patients
started on therapy within 3 h. Despite these trials, the practice of giving
high-dose steroids remains controversial as a result of increased morbidity and
early mortality.4,5,6,7
Also, in a small series of patients, the
administration of GM1 ganglioside (100 mg intravenously each day from the time
of the accident) was found to enhance ultimate recovery to a modest degree but
this finding has not been corroborated. GM1 ganglioside is a complex
carbohydrate and is a normal constituent of the neuronal cell membrane. GM1
ganglioside has been implicated in cell growth, development, and repair. Two
North American multicenter clinical trials have failed to demonstrate a
convincing benefit inpatients with acute SCI. The data suggest that patients
treated with GM1 ganglioside may improve faster than controls, but long-term
outcome has not been significantly different from that of control groups. For
those wishing to administer GM| ganglioside despite the lack of proven clinical
efficacy, the accepted protocol is methylprednisolone per the NASCIS II
protocol within 8 hours of injury, followed immediately by a loading dose of
GM1 ganglioside 300 mg and a maintenance dosage of 100 mg per day for 56 days.4,5,7
Surgical
Treatment
Surgical treatment is often necessary for
decompression and stabilization of the spine. Immediate surgical intervention
is warranted if the spine is unstable or evidence of cord compression exists
with an incomplete injury. Otherwise, the timing of intervention remains
controversial. The intent is to maintain an environment for optimal recovery of
the cord. Early surgery can remove bony fragments or herniated disc material
and stabilize the spine with severe ligamentous injury, which may cause
impingement upon the cord despite immobilization. In theory, early surgery is
done before worsening symptoms related to edema or hematoma occur. The argument
for late surgery (more than 7 d after injury) is to allow time for medical
management such as treatment of autonomic instability while at the same time
letting swelling subside. Patients undergoing early intervention often worsen
neurologically in the post-operative period related to swelling.4,5
The current available evidence suggests favorable
outcome for surgeries that are performed within 25 h or after 200 h of the
acute SCI . However, there are no studies of hyperacute SCI surgeries
(i.e., within 6 h of symptom onset). Traction may be required to decompress the
malaligned spine if early surgical intervention is not desired. Care must be
taken to avoid further injury with too much traction. MRI of the spine before
and after traction is recommended. In some cases the patient may heal without
an operation and these patients should be stabilized in a hard collar or halo
vest allowing for early mobilization and rehabilitation. Rarely is there any
improvement after a complete SCI making surgery at any time controversial.
Penetrating SCI from a knife, bullet, or other foreign object frequently does
not require surgery. Infection and the development of cerebrospinal fluid fistulae
would favor surgical exploration and possible debridement of the foreign body
tract. Broad-spectrum antibiotics such as a third generation cephalosporin
should be given for 2 wk after injury regardless of surgical intervention.4,5
b.
Chronic
medical management
Chronic medical therapy is directed
at preventing and treating the common, and often severe, medical complications
of SCI. SCI produces a wide variety of changes in systemic physiology that can
lead to a number of complications, which rival the impact of neurologic deficit
on function and quality of life. Rehospitalization occured in 53% of patinents
in first years after SCI and continued at a stable rate of about 37% per year
over the next 20 years. Factor contributing to the risk of rehospitalization
included increased agen and severity of SCI.6
Autonomic Dysreflexia
Distension
of the bladder or bowel in patients with SCI may lead to episodes ol autonomic
dysreflexia, a potentially life-threatening situation. Seen in patients with
SCI at the midthoracic level or above, the syndrome may also be triggered by
other noxious stimuli, including decubiti, nephrolithiasis, genitourinary
infection, surgical procedures under local anesthesia, and biliary disease. The
syndrome presents as chills, diaphoresis, piloerection, hypertension, reflex
bradycardia, pupillary dilatation, headache, and pallor. Death can result from
cardiac arrest caused by profound vagal overactivity. Symptoms can occur even
years after SCI. The Threshold for the development of symptoms appears to
decrease with successive episodes. Treatment should be directed at eliminating
the noxious stimulus (e.g., bladder or bowel decompression). Antihypertensive
agents or vagolytics (e.g., atropine) may be necessary in severe cases.5
Coronary
artery disease
Coronary artery disease (CAD) is a prominent
complication of SCI with long term survivors. Patients with SCI is mpre likelu
to acquire CAD risk factors than the average population secondary to loss of
muscle mass, inactivity, and increased body fat.6
Pulmonary
disease
Respiratory function must be closely monitored, as
respiratory embarrassment is the most common cause of morbidity and mortality
in SCI patients. During the initial examination the respiratory rate, oxygen
saturation, and the vital capacity should be recorded in addition to obtaining
a chest radiograph. Patients with C1-C3 injury are usually apneic after injury
and require mechanical ventilation. In patients not requiring instant
intubation, the vital capacity should be recorded several times daily. The most
frequent respiratory complications include atelectasis, pneumonia, pulmonary
edema, and respiratory failure. The treatment goal is to prevent these
complications. Several methods including aggressive pulmonary toilet, intermittent
positive pressure ventilation (IPPV), intrapulmonary percussion, cough
stimulation (with vibration using vest or vibrator, or cough stimulators), and
postural drainage (via clapping, position changes, or special positioning beds)
are used for prevention. Despite these measures, SCI patients often have
reduced cough and increased secretions, which may require mechanical
ventilation. Tracheostomy should be considered in patients during the first one
to two weeks even if the patient is considered a candidate for weaning in the
future.4
Genitourinary
complications
Urological
complications were significant causes of morbidity and mortality in the past,
but this is declining with improving current genitourinary care. Initial workup
after SCI should include a baseline blood urea nitrogen and creatinine, urine
analysis, and urine culture. The patient may have an indwelling foley catheter
after SCI. During this period of SS, the urinary bladder is atonic. This
usually lasts 6– 8 wk as mentioned previously, but may extend up to 1 yr. When
the patient is medically stable, intermittent catheterization should begin.
Routinely this is done every 4 h, but should be done more frequently for
increased fluid intake or when residual volumes are greater than 500 cc for two
consecutive measurements.4,6
Frequent
urological complications include urinary tract infection, pyelonephritis, renal
nephrolithiasis, and renal failure. These patients frequently have chronic
bacteruria, but should only be treated if symptoms of fever, flank pain,
purulent urine, or hematuria develop. Periodic urine cultures should be
obtained, so rapid treatment with the proper antibiotic can be initiated if the
patient becomes symptomatic. The best preventative measure is good hydration.
Other methods for prevention of infection such as chronic low dose antibiotic,
intravesical instillation of antibiotic, or the acidification of urine with
vitamin C have not proven to be beneficial, but still are used as standard of
care in SCI patients. Once aggressive medical management fails, then alternate
surgical options should be explored.4,6
Gastroinstestinal
dysfunction
Bowel
dysfunction is very common after SCI and can significantly affect quality of
life. There are no evidence based recommendations for clinical management of
this problem. A structured bowel regimen employing a regular diet, 2 – 3 L of
fluid/day, 30 g of fiber, and chemical and mechanical stimulation is often
employed to chieve predictable bowel evacuation to avoid fetal incontinence and
impaction.4,6
Spasticity
The increased tone
is beneficial for some activities such as sitting, standing, and even coughing,
but proves to be a hindrance for mobility during rehabilitation, activities of
daily living, and sleep. Initial treatment includes stretching of the limbs and
physical therapy. The next step involves adding pharmacologic agents. The most
common medications used are baclofen 10–200 mg daily in four divided doses,
diazepam 4–60 mg daily in three divided doses, dantrolene 25–400 mg daily in
three divided doses, clonidine 0.05 mg twice daily to 0.4 mg daily, or
tizanidine 2–36 mg daily.4,6
If the above medications fail, injections are
attempted such as peripheral nerve blocks, botulinum toxin, or motor point
blocks. Intrathecal baclofen can be delivered via an implanted pump. If these
therapeutic modalites fail, then other surgical options are explored. The most
common surgical procedure in SCI patients is dorsal root entry zone lesions
(DREZ-otomy) during which the lateral small pain fibers are destroyed reducing
the pain and spasticity.4,6
39. Prognosis
The greatest risk to
the patient with spinal cord injury is in the first 10 days when gastric
dilatation, ileus, shock, and infection are threats to life. According to
Messard and colleagues, the mortality rate falls rapidly after 3 months; beyond
this time, 86 percent of paraplegics and 80 percent of quadriplegics will
survive for 10 years or longer. In children, the survival rate is even higher
according to DeVivo and colleagues, who found that the cumulative 7-year
survival rate in spinal cord–injured children (who had survived at least 24 h
after injury) was 87 percent. 7
Chronic pain (present in 30 to 50 percent of cases)
requires the use of nonsteroidal anti-inflammatory medication, injections of local
anesthetics, and transcutaneous nerve stimulation. A combination of
carbamazepine or gabapentin and either clonazepam or tricyclic antidepressants
may be helpful in cases of burning leg and trunk pain. Recalcitrant pain may
require more aggressive therapy, such as epidural injections of analgesics or
corticosteroids or an implanted spinal cord stimulator that is applied to the
dorsal columns, but often even these measures are ineffective. Spasticity and
flexor spasms may be troublesome; oral baclofen, diazepam, or tizanidine may
provide some relief. In permanent spastic paraplegia with severe stiffness and
adductor and flexor spasms of the legs, intrathecal baclofen, delivered by an
automated pump in doses of 12 to 400 mg/day, has also been helpful. The drug is
believed to act at the synapses of spinal reflexes (Penn and Kroin). One must
always be alert to the threat of pulmonary embolism from deepvein thrombi,
although the incidence is surprisingly low after the first several months.
Physical therapy, muscle re-education, and the proper use of braces are all
important in the rehabilitation of the patient. All this is best carried out in
special centers for rehabilitation of spinal cord injuries.4,6,7
Basic
of Diagnosis
Clinic
diagnosis : Spinal
Shock
From Anamnesis and Physical Examination we get :
-
Paraparese with flaccid type (miotome L2 – S1)
-
Hipestesi dermatom L4-S3 in right and left leg with
parestesia
-
Abnormality of defecate and urination
-
Areflex of Patella reflex and achilless
reflex
Spinal Shock is the loss of motor function at the
time of injury—tetraplegia with lesions of the fourth to fifth cervical
segments or above, paraplegia with lesions of the thoracic cord—is accompanied
by immediate atonic paralysis of bladder and bowel, gastric atony, loss of
sensation below a level corresponding to the spinal cord lesion, muscular
flaccidity, and almost complete suppression of all spinal segmental reflex
activity below the lesion. As a result of their sudden separation from higher
levels, the neural elements below the lesion fail to perform their normal
function. Also impaired in the segments below the lesion is the control of
autonomic function. Urine accumulates until the intravesicular pressure is
sufficient to overcome the sphincters; then driblets escape (overflow
incontinence). There is also passive distention of the bowel, retention of
feces, and absence of peristalsis (paralytic ileus). Genital reflexes (penile
erection, bulbocavernosus reflex, contraction of dartos muscle) are abolished or
profoundly depressed.7
Topic Diagnosis : anterior cord of L2
medulla spinalis
When
the spinal cord is suddenly and virtually or completely severed, three
disorders of function are at once evident: (1) all voluntary movement in parts
of the body below the lesion is immediately and permanently lost; (2) all
sensation from the lower (aboral) parts is abolished; and (3) reflex functions
in all segments of the isolated spinal cord are suspended.7
We
can conclude TD from clinical manifestations and physical exam:
-
Paraparese with flaccid type (L2 – S1)
-
Hipestesi dermatom L4-S3 in right and left leg with
parestesia
-
Abnormality of defecate and urination
-
Areflex of Patella reflex and achilless
reflex
In the anterior cord syndrome, The dorsal
columns are spared, but the corticospinal and spinothalamic tracts are
compromised. The clinical symptoms include a loss of motor function, pain
sensation and temperature sensation at and below the injury level with
preservation of light touch and joint position sense.8
ETIOLOGIC DIAGNOSIS : Trauma Vetebrae
From the anamnesis, we get that that
patient had history of trauma, patient
fell from roof with height approximately ± 5 m. Patient fell from the roof when
repaired the tile with supine position which the back first get the trauma and
lifted the head upward.
From anamnesis and physical examination,
we considered the lesion in anterior cord of medulla spinalis (L2) so the
radiological sentration it would be higher on vertebrae segment. The vertebrae
segment can caused that lesion it must be on vertebra thoracal 11/12. So the
radiological sentration we suggest must on Ro. Thoraco-Lumbal (ap-lateral).
DIFFERENTIAL DIAGNOSIS:
Radicular injury
The complete spinal cord transection syndrome is most commonly
caused by trauma, less commonly by inflammation or infection (transverse
myelitis). Acute spinal cord trauma initially produces so-called spinal
shock, a clinical picture whose
pathophysiology is incompletely understood. Below the level of the lesion there
is complete, flaccid paralysis, and all modalities of sensation are lost.
Bladder, bowel, and sexual function are lost as well. Only the bulbocavernosus
reflex is preserved—an important point for the diagnostic differentiation of
this condition from polyradiculitis, in which it is typically absent.1
References
11. Rokhamm R. Colour atlas of neurology. Thieme.
Stuttgart, Germany. 2004. h 30-33; 272-275;
380-381
22. Baethr
M, Frotscher M. Duus: Topical diagnosis in neurology: Anatomy
· Physiology · Signs · Symptoms. 4th completely
revised edition. New York. Thieme. h 23-30.
33. Selections
from the Netter Collection of Medical Illustrations. Atlas of neuroanatomy and
neurophysiology. Special Edition. USA. Icon Custom Communications. 2002. h 22
44. Derwenskus J, Zaidat O.O. Spinal Cord
Injury and Related Diseases. Dalam Suarez J.L, editors. Critical care of neurology
and neurosurgery. Totowa, New Jersey. Humana Press. 2004. h 417-432.
55. Santiago P, Fessler R.G. Spinal Cord
Trauma. Dalam Bradley R.G et al, editor. Neurology in clinical practice:
principle of diagnosis and management. Fourth edition. Philadelphia. USA. An
imprint Elsevier.h 1150-1175.
66. Mandigo C.E, Kaiser M.G, Angevine P.D.
Spine Injury. Dalam: Rolowand L.P, Pedley T,A, editor. Merrit’s Neurology
Twelfth edition. Philadelphia. USA. LIPPINCOTT WILLIAMS & WOLTERS KLUWER
business. 2010. h 495-502.
77. Ropper A.H, Brown R.H. Adams and victor’s
principles of neurology. Eight Edition. New York. McGraw-Hill Companies MEDICAL PUBLISHING DIVISION. 2005. h
1049-1055.
88. International
standards for neurological classification of spinal cord injury (Revised 2011)











No comments:
Post a Comment
terima kasih sudah membaca blog saya, silakan tinggalkan komentar